62+ calculating reaction free energy under nonstandard conditions
Web The change in Gibbs free energy under nonstandard conditions ΔG can be determined from the standard change in Gibbs free energy ΔG⁰. Web About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact.
Solved How Do You Calculate The Change In Free Energy For A Reaction Under Nonstandard Conditions
Web How do you calculate the change in free energy for a reaction under nonstandard conditions.
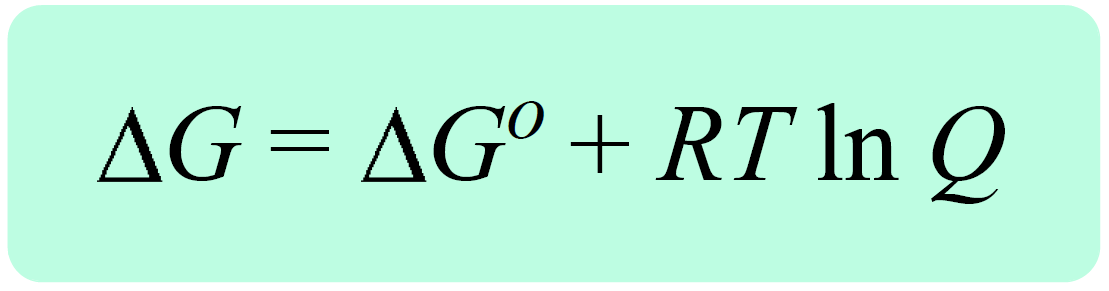
. Web In case one calculates enthalpy of water at 120oC he will have to perform integration both for liquid and gas phases in their respective regions of stability and then. Δ G Δ G ⁰ RT ln Q where R is. Using the appendix data to calculate the standard.
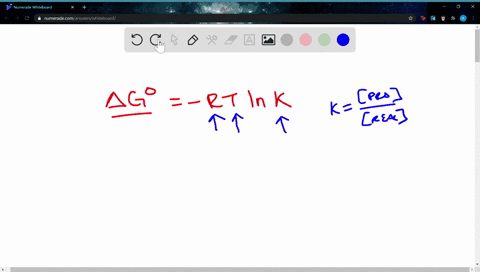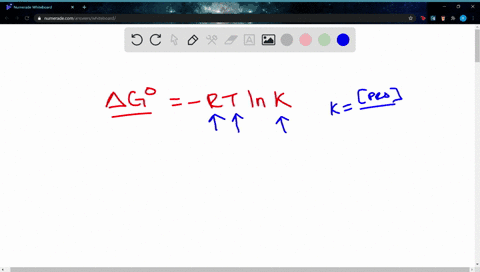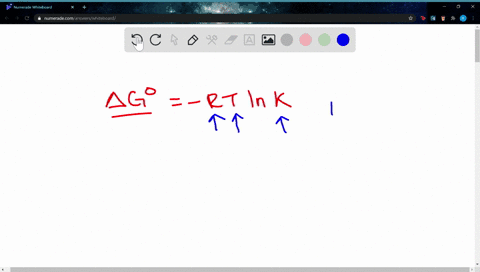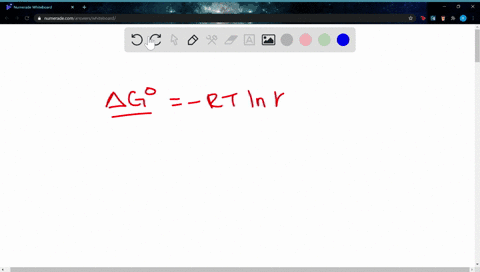
Δ G Δ G ⁰ RT ln Q where R is. Web If the reaction is not occurring under these standard conditions we need to use the Gibbs free energy equation in a modified form that takes into account the actual. Web The change in Gibbs free energy under nonstandard conditions ΔG can be determined from the standard change in Gibbs free energy ΔG⁰.
Web The change in Gibbs free energy under nonstandard conditions ΔG can be determined from the standard change in Gibbs free energy ΔG⁰. Δ G Δ H T Δ S From Appendix G. How well do the values of ΔG calculated this way compare to those.
A ΔGrnxΔGrnxRTlnQ B ΔGrnxΔGrnxRTlnQ C. Web Calculate E_cell for the reaction under the following nonstandard conditions and decide whether the reaction will occur spontaneously. Web If we know the standard free energy change for a process G o and the reaction quotient Q for the change we can determine the non-standard state free energy change G for.
Web 64Use standard free energies of formation to calculate ΔG at 25 C for each reaction in Problem 62. Web 182e Calculating reaction free energy under nonstandard conditions - YouTube AboutPressCopyrightContact. Δ G Δ G ⁰ RT ln Q where R is.
If a reaction is spontaneous under conditions it is. Web Calculating the Free-Energy Change under Nonstandard conditions Which of the following statements is true. Web The standard change in free energy may be calculated using the following equation.
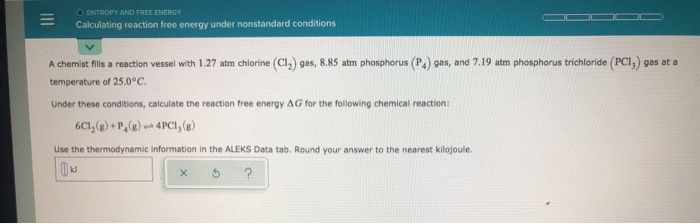
Solved O Entropy And Free Energy Calculating Reaction Free Chegg Com
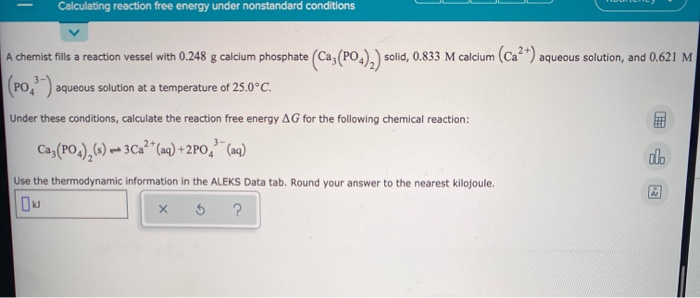
Calculating Reaction Free Energy Under Nonstandard Chegg Com
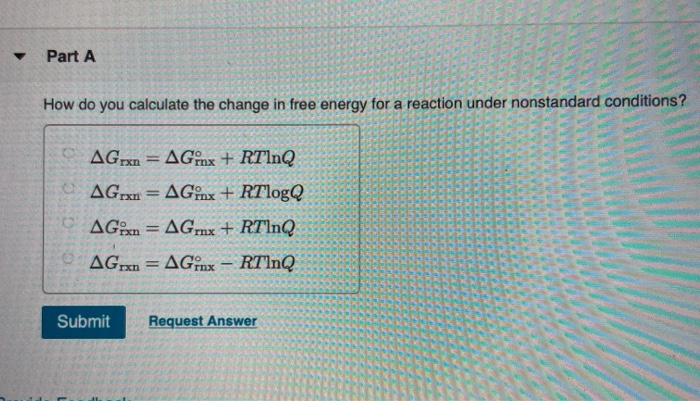
Solved Part A How Do You Calculate The Change In Free Energy Chegg Com

Aleks Calculating Reaction Free Energy Under Nonstandard Conditions Youtube
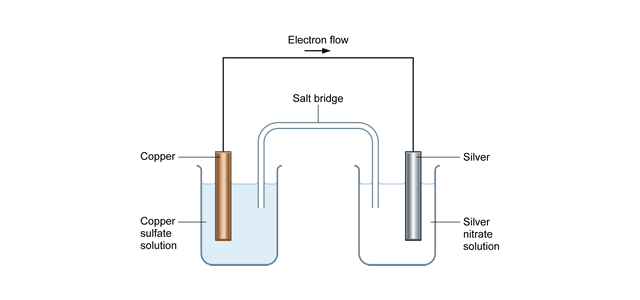
The Gibbs Free Energy Post 16 Thermodynamics Tutorials Resource Rsc Education
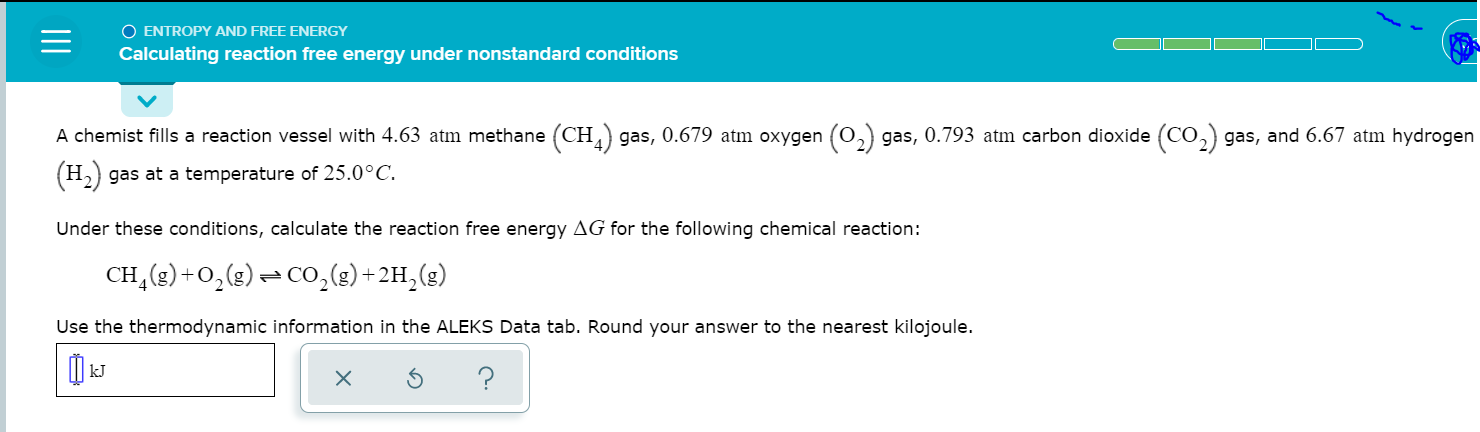
Solved O Entropy And Free Energy Calculating Reaction Free Chegg Com

Solved R The Model Standard And Instantaneous Gibbs Free Chegg Com

Free Energy And Cell Potential Video Khan Academy
Gibbs Free Energy And The Spontaneity Of Chemical Reactions

Calculate Gibbs Free Energy Change For A Reaction At Elevated Temperature Youtube

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy

Calculating Standard Free Energy Changes Video

Gibbs Free Energy Wikipedia
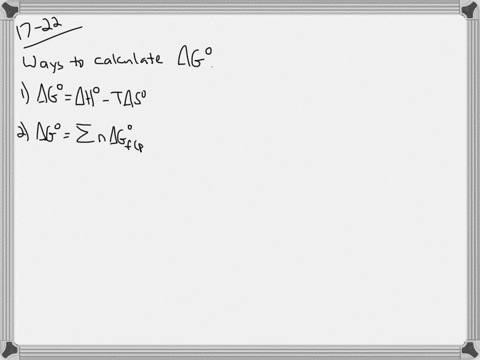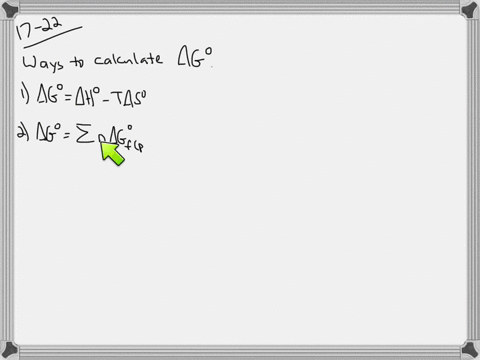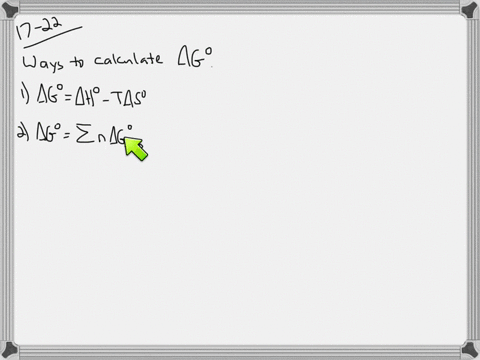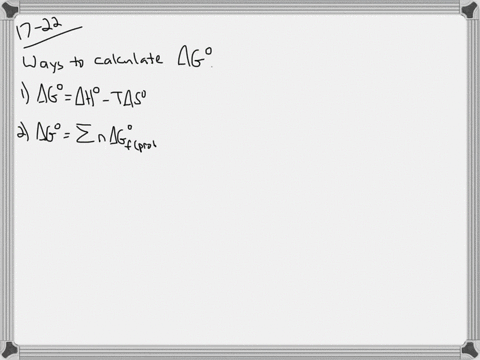
Solved How Do You Calculate The Change In Free Energy For A Reaction Under Nonstandard Conditions

The Relationship Between Free Energy And The Equilibrium Constant Video Lesson Transcript Study Com

Free Energy Changes In Biochemical Systems

Calculating Reaction Free Energy Under Nonstandard Conditions副本 16 12 4 上午 1 37 Aleks 第 1 共 4 Course Hero